About
COMIRNATY
COMIRNATY: The First Approved COVID-19 Vaccine1,2
COMIRNATY is an mRNA COVID-19 vaccine approved for use in
individuals 12 years of age and older to help protect against
COVID-191
Researchers have been studying and working with mRNA technologies for other diseases for decades3,4
The pivotal study (Study 2) of COMIRNATY was one of the largest clinical trials Pfizer has ever conducted1
- It is the main study to support the safety and efficacy of COMIRNATY
- A multicenter, multinational, randomized, placebo-controlled, double-blind, efficacy (Phase 2/3) study in a diverse population of approximately 46,000 people 12 years of age or older, of which approximately 22,000 received COMIRNATY
Multiple real-world studies on different formulas of COMIRNATY have been published and are generally supportive of the effectiveness of COMIRNATY against COVID-19 and severe disease5,6
The results from one real-world study associated with the 2023-2024 Formula of COMIRNATY are presented in the Real-World Evidence tab below
COMIRNATY:
The first
FDA-approved,
mRNA-based
COVID-19 vaccine2
In development since the 1960s, mRNA technology has been explored across various diseases. Studies evaluating use of mRNA as a vaccine platform began in the 1990s3,4,7,8
mRNA technology, used by mRNA COVID-19 vaccines including
COMIRNATY, has
the potential to streamline vaccine
development.
This helps to allow for a timely response to FDA-directed formula
updates/strain(s) selection for circulating strains of the virus
that causes
Updating the formula of
How COMIRNATY works
COMIRNATY helps train the immune system to recognize and help fight the virus that causes
-
1.
COMIRNATY contains a spike protein mRNA segment surrounded by a protective lipid coat for delivery. After vaccination, the mRNA enters the cells.4,11
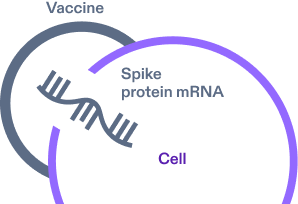
-
2.
mRNA provides the cell instructions to build copies of spike proteins found on the surface of the virus that causes
COVID-19.4,11 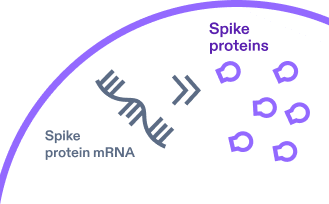
-
3.
The spike proteins are presented on the surface of the cell and train the immune system. The resulting immune response, which includes antibodies, is what recognizes and helps protect against
COVID-19 if the body is exposed again in the future.4,11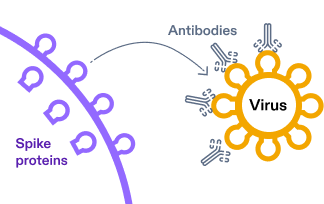
Note: These graphical representations are intended for illustrative purposes only.4,11
Clinical Trial Overview
Supporting the development of COMIRNATY (COVID-19 Vaccine, mRNA)
COMIRNATY overview

Actor portrayals.
COMIRNATY
overview
- COMIRNATY effectiveness and safety have been evaluated in multiple randomized controlled trials1
- Analyses of prespecified immunogenicity endpoints were also studied1
- Pfizer is committed to ongoing monitoring of the safety and effectiveness of our vaccines1
- The safety and immunogenicity data of previous formulas of COMIRNATY are pertinent to the 2024-2025 Formula of COMIRNATY because previous formulas of the vaccine are manufactured using the same process, with differences only in the mRNA that encodes the viral spike proteins1
- COMIRNATY should not be administered to individuals with a known history of severe allergic reaction (e.g., anaphylaxis) to any component of COMIRNATY. Appropriate medical treatment must be immediately available to manage potential anaphylactic reactions following administration of COMIRNATY1
- Postmarketing data with authorized or approved mRNA COVID-19 vaccines demonstrate increased risks of myocarditis and pericarditis, particularly within the first week following vaccination. For COMIRNATY, the observed risk is highest in males 12 through 24 years of age1
- Syncope (fainting) may occur in association with administration of injectable vaccines, including COMIRNATY. Procedures should be in place to avoid injury from fainting. Immunocompromised persons, including individuals receiving immunosuppressant therapy, may have a diminished immune response to COMIRNATY1
More information
Read the full Prescribing Information for additional information on
effectiveness,
immunogenicity, and safety of COMIRNATY
COMIRNATY Pivotal Trial Data
(Study 2)
About COMIRNATY pivotal trial
(Study 2)
The efficacy and safety of a
Note: Data from clinical studies were added to the full Prescribing Information in 2023 to support use of a single dose of COMIRNATY in individuals
≥12 years of age.1
Over 46,000 participants 12 years of age and older
were randomized to COMIRNATY or placebo
in the pivotal trial (Study 2)1
Study design
Study 2 is a phase 1/2/3 multinational, multicenter,
1:1 randomized,
Study objectives1
- The study evaluated vaccine efficacy against confirmed symptomatic COVID-19,* and in a subset of participants, as a secondary endpoint, severe COVID-19
- Safety was also assessed, based on solicited specific local or systemic adverse events, and unsolicited adverse events and serious adverse events
Study population1
- Approximately 46,000 participants
-
16 years of age and older†
(COMIRNATY, n=22,026; placebo, n=22,021) -
12-15 years of age‡
(COMIRNATY, n=1,131; placebo, n=1,129)§∥
Select inclusion criteria1,12
- ≥12 years of age
- Participants who were healthy or had a stable chronic medical condition, including those with HIV, HCV, or HBV
-
Participants who were, in the judgment of the investigator, at
risk of contracting COVID-19
Select exclusion criteria1,12
-
Previous clinical diagnosis of COVID-19 based on COVID-19
symptoms/signs alone, if a SARS-CoV-2 NAAT result
was not available -
Previous microbiological diagnosis of COVID-19 based on COVID-19
symptoms/signs and a positive SARS-CoV-2
NAAT result - Participants who were immunocompromised
SARS-CoV-2 variants of concern1
-
Identified from COVID-19 cases for the 16 years of age and
older group; included B.1.1.7 (Alpha) and B.1.351 (Beta)
Intervention1
-
Two doses of COMIRNATY containing 30 mcg of modRNA encoding the spike
protein of the Wuhan-Hu-1 strain of
SARS-CoV-2
*The COMIRNATY pivotal study took place when much of the US population had not yet been infected with or vaccinated against SARS-CoV-2.1,13
†At the time of the analysis of the ongoing study with a data
cutoff of March 13, 2021, a total of
‡At the time of the analysis of the ongoing study with a data cutoff of September 2, 2021, there were 1,559 adolescents 12 to 15 years of age followed for ≥4 months after the second dose.1
§Participants were also monitored for unsolicited adverse events throughout
the study
∥Participants were planned to be followed for up to 24 months, for assessments of safety and efficacy against COVID-19.1
HBV=hepatitis B virus; HCV=hepatitis C virus; HIV=human immunodeficiency virus.
Study 2 Efficacy
Primary series with COMIRNATY—
Efficacy in participants
16 years of age and older
Primary efficacy endpoint: First COVID-19 occurrence from 7 days after
dose 2
in participants 16 years of age and older1
91.1% vaccine efficacy¶ (95% CI: 88.8, 93.1) among participants without evidence of prior SARS-CoV-2 infection (COMIRNATY, n=19,711)1
16-64 years of age
90.5% vaccine efficacy¶
(95% CI: 87.9, 92.7)
(COMIRNATY, n=15,519)1
94.5% vaccine efficacy¶
(95% CI: 88.3, 97.8)
(COMIRNATY, n=4,192)1
90.9% vaccine
efficacy¶ (95% CI: 88.5, 92.8) among
participants
with or without evidence of
prior
SARS-CoV-2 infection (COMIRNATY,
n=20,533)1

IMPORTANT NOTE: The vaccine effectiveness noted above was evaluated in the clinical trial. Vaccine effectiveness will naturally wane over time and may also be impacted by the emergence of new variants.10
¶Vaccine encoding the viral spike (S) glycoprotein of SARS-CoV-2 Wuhan-Hu-1 strain (Original).1
Secondary efficacy
endpoint: First severe COVID-19
occurrence from
7 days after dose 2
in participants
16 years of age and older1
95.3% vaccine efficacy (95% CI: 70.9, 99.9) for first
severe COVID-19 occurrence
(severe disease as defined in the protocol),#
with or without evidence of prior
SARS-CoV-2 infection (COMIRNATY,
N=20,540)1
100% vaccine efficacy (95% CI: 87.6, 100) for first severe COVID-19 occurrence (severe disease as based on the CDC definition),** with or without evidence of prior SARS-CoV-2 infection (COMIRNATY, N=20,513)1

IMPORTANT NOTE: The vaccine effectiveness noted above was evaluated in the clinical trial. Vaccine effectiveness will naturally wane over time and may also be impacted by the emergence of new variants.10
#Defined as confirmed COVID-19 and ≥1 of the following: clinical signs at rest indicative of severe systemic illness, respiratory failure, evidence of shock, significant acute renal, hepatic, or neurological dysfunction, admission to an intensive care unit, or death.1
**Defined as confirmed COVID-19 and ≥1 of the following: hospitalization, admission to an intensive care unit, intubation or mechanical ventilation, or death.1
Notes
- The vaccine efficacy was observed during a period of time before the Omicron variant was the predominant strain of SARS-CoV-21,14
- SARS-CoV-2 variants of concern identified from COVID-19 cases from this data cutoff included B.1.1.7 (Alpha) and B.1.351 (Beta)1
- Vaccine effectiveness will naturally wane over time and may be impacted by the emergence of new or different variants10
- Confirmed cases were determined by reverse transcription polymerase chain reaction (RT-PCR) and symptom(s) including fever, new or increased cough, new or increased shortness of breath, chills, new or increased muscle pain, new loss of taste or smell, sore throat, diarrhea, or vomiting1
More information
To learn about the clinical study data supporting the safety and effectiveness of COMIRNATY in individuals 12 through 15 years of age
See Prescribing InformationSelect safety data from clinical studies
An overview of clinical studies contributing to the safety assessment of COMIRNATY1
Participants in these clinical studies received a 2-dose series, 3 weeks
apart
(referred to as a primary series)
and
subsequent doses referred to as booster doses1
| << swipe left | << swipe >> | << swipe >> | swipe right >> | ||||
|---|---|---|---|---|---|---|---|
| Study | Number of Participants | Participant Age | Dose |
|
|||
| Primary Series | |||||||
|
Study 1 (NCT04380701) |
60 | 18 years through 55 years | Primary Series | Originala | |||
|
Study 2 (NCT04368728) |
1,131b | 12 years through 15 years | Primary Series | Originala,b | |||
| 22,026b | ≥16 years | ||||||
| Booster Dose | |||||||
|
Study 2 (NCT04368728) |
825 | 12 years through 15 years | 1st Booster | Originala,b | |||
| 306 | 18 years through 55 years | ||||||
|
Study 4 (NCT04955626) |
65 | 12 years through 17 years | 1st Booster | Originala,b | |||
| 5,081 | ≥16 years of age | ||||||
|
Study 5 (NCT05472038) |
726 | ≥12 years | 2nd Booster | Originala,c and Bivalent | |||
| Concomitant Administration | |||||||
|
Study 8 (NCT05310084) |
1,128 | 18 years through 64 years | 2nd Booster administered alone or concomitantly with Influenza Vaccined | Originala,d | |||
aCOMIRNATY encoding the viral spike (S) glycoprotein of SARS-CoV-2 Wuhan-Hu-1 strain (Original).1
bReceived COMIRNATY during placebo-control period.1
cVaccine encoding the viral spike (S) glycoprotein of
SARS-CoV-2 Wuhan-Hu-1 strain (Original) and Omicron
variant lineages BA.4 and BA.5 (Omicron BA.4/BA.5), authorized as
Pfizer-BioNTech COVID-19 Vaccine, Bivalent.1
dInfluenza Vaccine (Afluria Quadrivalent).1
The safety of a 2-dose primary series of the original monovalent
COMIRNATY was evaluated in Study 21
An overview of most commonly reported adverse reactions (≥8 and ≥10) following any dose in participants receiving a 2-dose primary series of COMIRNATY1
Because clinical trials are conducted under widely varying conditions,
adverse reaction rates observed in the clinical trials of a vaccine
cannot be directly compared to rates in the clinical
trials of another vaccine and may not reflect the rates observed in
practice1
| << swipe left | << swipe >> | swipe right >> | |||
|---|---|---|---|---|---|
| Reaction, % participants | 12-15 yearsa | 16-55 yearsb | 56+ yearsb | ||
| Local reactions | |||||
| Pain at the injection site | 90.5% | 88.6% | 78.2% | ||
| Injection site swelling | 9.2% | 10.6% | 11.8% | ||
| Injection site redness | 8.6% | - | 10.4% | ||
| Systemic reactions | |||||
| Fatigue | 77.5% | 70.1% | 56.9% | ||
| Headache | 75.5% | 64.9% | 45.9% | ||
| Chills | 49.2% | 41.5% | 24.8% | ||
| Muscle pain | 42.2% | 45.5% | 32.5% | ||
| Fever | 24.3% | 17.8% | 11.5% | ||
| Joint pain | 20.2% | 27.5% | 21.5% | ||
aMost commonly reported adverse reactions ≥8%.1
bMost commonly reported adverse reactions ≥10%.1
Real-World Evidence
Select real-world analysis of vaccine effectiveness associated with COMIRNATY (COVID-19 Vaccine, mRNA)
 Actor
Actorportrayal.
Randomized controlled trials (RCTs) and real-world evidence (RWE) analyses
Together, randomized controlled trials and real-world evidence may help inform healthcare clinical practice15-20
RCTs: Gold Standard
Interventional studies
- Gold standard for evaluating efficacy and safety of new drug treatments according to the FDA15,16
- Designed to eliminate systematic biases when comparing drug treatments15
Limitations
- Due to highly specific inclusion and exclusion criteria, RCT results may not be generalizable to the broader patient population in clinical practice16
- May not be able to address questions that require large patient populations or when ethical or time constraints exist15,16
RWE: May Complement RCT Findings
Observational, non-interventional
- May use data from routine clinical practice17
- RWE is clinical evidence about the usage and potential benefits or risks of a medical product derived from analysis of real-world data. Real-world data are data related to patient health status and/or delivery of health care17,18
- May include a broader, more heterogeneous patient population and, therefore, may be more generalizable15,17,19,20
Limitations
- Unable to determine causality17,20
- Biases related to treatment selection and unobserved variables cannot be fully addressed15,17
- Other limitations include limited internal validity, potential data quality, and/or methodology issues15,17,19,20
Observational RWE analyses are not intended for direct comparison with clinical trials and may introduce bias.17,19
 Actor
Actorportrayal.
Helping understand absolute, relative, and incremental vaccine effectiveness (VE)21
- Absolute VE compares frequency of health outcomes in vaccinated and unvaccinated individuals
- Relative VE compares frequency of health outcomes in individuals who received one type of vaccine to individuals who received a different vaccine type
- Incremental VE compares frequency of health outcomes in individuals who received more doses with those who received fewer doses

the 2023-2024 respiratory virus season.
This study has several limitations that healthcare professionals should consider in evaluating results to
potentially complement RCT data:
-
The primary analysis included the use of healthcare encounter
data from a single healthcare system.
Reliance on data from one healthcare system may limit external validity due to potential biases or lack of heterogeneity of study populations - Residual Confounding from Test Negative Design: Differences in exposure risk and disease severity between vaccinated and unvaccinated individuals may not have been fully accounted for, potentially impacting the study's conclusions on vaccine effectiveness
-
Short Follow-Up Period: The median follow-up after
receiving a 2023-2024 Formula COMIRNATY vaccination was 58 days,
which may not capture long-term vaccine effectiveness or associated
durability
of protection - Potential misclassification of a patient hospitalized with a positive SARS-CoV-2 test as being hospitalized for severe COVID-19 when SARS-CoV-2 infection was incidental to but not the reason for hospitalization. Inclusion of patients hospitalized for other reasons can result in underestimation of VE
- Missing genotype information and potential for misclassification of SARS-CoV-2 sublineages: Lack of complete genotype data or misclassification can limit the ability to differentiate and assess the vaccine's effectiveness against specific SARS-CoV-2 sublineages
- Information on Prior Infection: Misclassification of previous infections, particularly unreported or undocumented cases due to at-home testing or decreases in testing rates, potentially affecting the reliability of study results. Limited data on prior infections and high rates of prior SARS-CoV-2 infection may confound vaccine effectiveness estimates
-
COMIRNATY Uptake: Low vaccination rates with
COMIRNATY among participants, or vaccination rates with significant
differences between groups (eg, elderly or individuals with
comorbidities), may impact results, potentially impacting
generalizability
and resulting in underestimation of vaccine effectiveness - Misclassification of Vaccination Status: There is a risk of errors in documenting previous COVID-19 vaccination status, which could affect study results
RWE Analysis: Estimated vaccine effectiveness of
the XBB.1.5–adapted 2023-2024 Formula of
COMIRNATY among adults ≥18 years of age
(October 10, 2023–February 29, 2024)5,6
Hospitalization is a widely recognized measure of severe
COVID-1921; other analyses of COVID-19–associated outcomes
are available in the publication not shown here
Note: Vaccine effectiveness will
naturally wane over time and may also be
impacted by the emergence of new variants.10
VE findings should be interpreted as the incremental benefit provided by
COVID-19 vaccination in a population with
a high prevalence of vaccine- and infection-induced immunity at the
start of the 2023-2024 respiratory virus season.22,23
Study design
| Study design | Main measures |
|---|---|
| Population6 |
7,572 acute respiratory infection encounters in adults ≥18 years of age (median age: 56 years)
|
| Control group6 |
52,036 acute respiratory infection encounters in adults ≥18 years of age (median age: 56 years)
|
| Data source5,6 | Kaiser Permanente Southern California |
| 2023-2024 Formula5,6 | Omicron XBB.1.5–adapted vaccine* |
| Study period6 |
October 10, 2023–February 29, 2024 |
| Median time since vaccination6 | 58 days (range: 15-156 days) |
| Circulating variants5,6 |
XBB sublineages were predominant with JN.1 rapidly increasing in prevalence |
| Design5,6 |
A test-negative, case-control study |
| Funding/support5 | Pfizer |
*Previous formulas of COMIRNATY are no longer available for use.1
Notes
- “Estimated effectiveness of the BNT162b2 XBB vaccine against COVID-19” by Tartof et al was a test-negative, case-control study that evaluated estimates of the COMIRNATY 2023-2024 Formula against COVID-19–associated hospital admissions and ED or UC encounters5
- This test-negative, case-control vaccine effectiveness (VE) analysis assessed the VE against XBB and JN.1 sublineages of COVID-19 during the 2023-2024 season5,6
- Cases included those presenting with an acute respiratory illness who had a positive SARS-CoV-2 PCR test; while controls had an acute respiratory illness but tested negative for SARS-CoV-2 (and had no encounters, in any setting, with a positive SARS-CoV-2 test in the prior 90 days)5,6
- Hospitalization is a widely recognized measure of severe COVID-19. The limitations of this measure as a marker of severe disease include the challenge in distinguishing hospitalizations due to COVID-19 from hospitalizations in which SARS-CoV-2 infection is incidentally identified, which can lead to differences in vaccine effectiveness estimates generated across various platforms21
Overall, limitations of an RWE analysis provide important contextual information and highlight the need for cautious interpretation of findings, as limitations may affect the reliability and generalizability of vaccine effectiveness estimates. VE findings should be interpreted as the added benefit provided by COVID-19 vaccination in a population with a high prevalence of vaccine- and infection-induced immunity at the start of the 2023-2024 respiratory virus season.
This study has several limitations that healthcare professionals should consider in evaluating results to potentially complement RCT data:
- The primary analysis included the use of healthcare encounter data from a single healthcare system. Reliance on data from one healthcare system may limit external validity due to potential biases or lack of heterogeneity of study populations
- Residual Confounding from Test Negative Design: Differences in exposure risk and disease severity between vaccinated and unvaccinated individuals may not have been fully accounted for, potentially impacting the study's conclusions on vaccine effectiveness
- Short Follow-Up Period: The median follow-up after receiving a 2023-2024 Formula COMIRNATY vaccination was 58 days, which may not capture long-term vaccine effectiveness or associated durability of protection
- Potential misclassification of a patient hospitalized with a positive SARS-CoV-2 test as being hospitalized for severe COVID-19 when SARS-CoV-2 infection was incidental to but not the reason for hospitalization. Inclusion of patients hospitalized for other reasons can result in underestimation of VE
- Missing genotype information and potential for misclassification of SARS-CoV-2 sublineages: Lack of complete genotype data or misclassification can limit the ability to differentiate and assess the vaccine's effectiveness against specific SARS-CoV-2 sublineages
- Information on Prior Infection: Misclassification of previous infections, particularly unreported or undocumented cases due to at-home testing or decreases in testing rates, potentially affecting the reliability of study results. Limited data on prior infections and high rates of prior SARS-CoV-2 infection may confound vaccine effectiveness estimates
- COMIRNATY Uptake: Low vaccination rates with COMIRNATY among participants, or vaccination rates with significant differences between groups (eg, elderly or individuals with comorbidities), may impact results, potentially impacting generalizability and resulting in underestimation of vaccine effectiveness
- Misclassification of Vaccination Status: There is a risk of errors in documenting previous COVID-19 vaccination status, which could affect study results
Observed estimated vaccine effectiveness
of 2023-2024 Formula of COMIRNATY† against
COVID-19 hospital admission5,6
In those who received XBB.1.5–adapted
2023-2024 Formula
of COMIRNATY† vs those
who did not
receive an
XBB.1.5–adapted† vaccine
of any kind5,6
Median time since vaccination: 58 days (range: 15-156 days)6
Note: Due to high rates of prior infection, VE estimates are evaluated as an incremental or relative benefit beyond what individuals may have from prior infection(s), vaccination(s), or both (hybrid immunity).24
†Those who did not receive an XBB.1.5–adapted vaccine included participants who received wild-type boosters, variant-adapted vaccines (eg, BA.1 or BA.4/5 bivalent vaccines), or unvaccinated individuals.5
NOTE: Vaccine effectiveness will
naturally wane over time and may also be impacted by the
emergence of new variants.10
This select real-world analysis of 2023-2024 Formula of COMIRNATY included the following publications5,6:
The interim analysis
was published in the Journal of the
American Medical Association and covered
the time period from October 10, 2023, to
December 10, 2023.5
[Tartof SY, Slezak JM, Frankland TB, et al. Estimated effectiveness of the BNT162b2 XBB vaccine against COVID-19. JAMA Intern Med. 2024;184(8):932-940. doi:10.1001/jamainternmed.2024.1640]
The full analysis
was published in Open Forum Infectious Diseases and covered the time period from October 10, 2023, to February 29, 2024.6
[Tartof SY, Slezak JM, Puzniak L, et al. Effectiveness of BNT162b2 XBB vaccine against XBB and JN.1 sublineages. Open Forum Infect Dis. 2024;11(7):ofae370. doi:10.1093/ofid/ofae370]
Note: Numerous studies and analyses have been conducted to evaluate the effectiveness of COVID-19 vaccines, yielding varying results across different populations, settings, and study designs. These variations may be attributed to differences in factors such as the timing of vaccine administration, the emergence of new viral variants, and demographic characteristics of study participants. Healthcare professionals should consider the context and specific conditions of individual studies when interpreting vaccine effectiveness data.5,21,25,26
Ordering available through Pfizer Prime*
Orders for 2024-2025 formula COVID-19 vaccines by BioNTech and Pfizer can be placed by eligible
healthcare professionals directly with Pfizer through Pfizer Prime online or by calling
*Eligible healthcare providers can order COVID-19 vaccines directly from
Pfizer. If preferred, orders may be placed with your facility’s wholesaler.